ALKANE THEORY
- Details
- Germán Fernández
- ALKANE THEORY
- Hits: 1236
What are alkanes?
Alkanes are compounds formed exclusively by carbon and hydrogen (hydrocarbons), which only contain simple carbon-carbon bonds.
Types of alkanes
Alkanes are classified as linear, branched, cyclic, and polycyclic.
Alkanes nomenclature
Alkanes are named by ending in -ane the prefix that indicates the number of carbons in the molecule (methane, ethane, propane...)
Physical properties of alkanes
The melting and boiling points of alkanes are low and increase as the number of carbons increases due to interactions between molecules by London forces. Linear alkanes have higher boiling points than their branched isomers.
Conformational isomers
Alkanes are not rigid due to the spin around the C-C bond. The multiple shapes created by these rotations are called conformations.
Newman projection
The energy of the different conformations can be seen in the Newman projections. Thus, in the case of ethane, the eclipsed conformation is the one with the highest energy, due to the repulsions between hydrogens.
Potential energy diagrams
The different conformations of alkanes can be represented in a potential energy diagram, where we can see which conformation is more stable (minimum energy) and the energy needed to go from one conformation to another.
Combustion of alkanes
Given their low reactivity, alkanes are also called paraffins. The most important reactions of this group of compounds are radical halogenations and combustion. Combustion is the combination of hydrocarbon with oxygen to form carbon dioxide and water.
- Details
- Germán Fernández
- ALKANE THEORY
- Hits: 239122
Hydrocarbons are compounds that contain only carbon and hydrogen. They are divided into two classes: aliphatic and aromatic hydrocarbons.
Aliphatic hydrocarbons include three classes of compounds: alkanes, alkenes, and alkynes. Alkanes are hydrocarbons that contain only carbon-carbon single bonds, alkenes contain carbon-carbon double bonds, and alkynes are hydrocarbons that contain one triple bond. 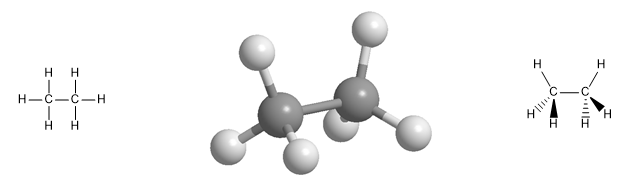
Molecular model of the ethane molecule. Ethane has a tetrahedral geometry, with carbon at the center of the tetrahedron and hydrogens oriented towards its vertices. Note the spatial representation with wedges and dashed lines .
- Details
- Germán Fernández
- ALKANE THEORY
- Hits: 177935
Alkanes are compounds made up of carbon and hydrogen that only contain carbon-carbon single bonds. They comply with the general formula CnH2n+2 , where n is the number of carbons in the molecule.
Alkanes in which the carbons are linked continuously (without branches) are called straight-chain alkanes .
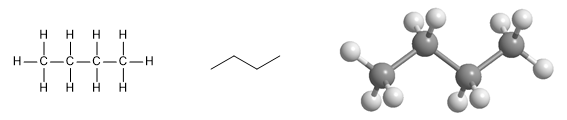
Butane molecule. The first drawing shows the form of union of the atoms, in the second the zig-zag shape of the molecule and finally its molecular model, where the spatial arrangement of the atoms is observed.
- Details
- Germán Fernández
- ALKANE THEORY
- Hits: 361560
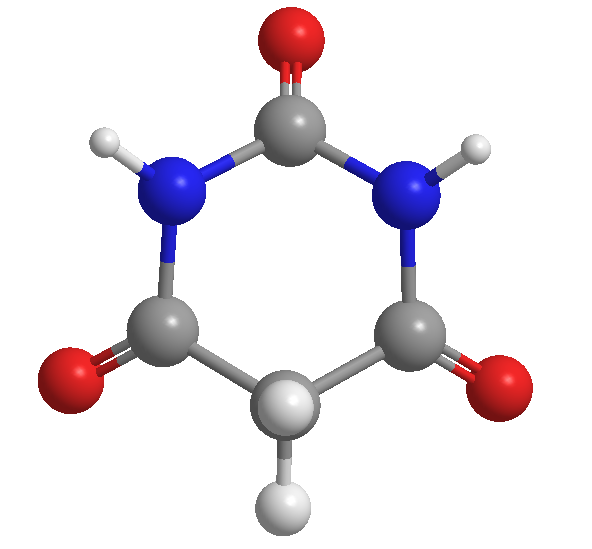
Barbituric acid, discovered by Adolf von Baeyer, in 1864
In the origins of chemistry, organic compounds were named by their discoverers. Urea gets its name from being isolated from urine.
Barbituric acid was discovered by the German chemist Adolf von Baeyer in 1864. It is speculated that he gave it this name in honor of a friend named Barbara.
Chemical science was advancing and the large number of organic compounds discovered made it essential to use a systematic nomenclature.

[1] Isobutane (common name); Methylpropane (IUPAC name)
[2] Isopentane (common name); Methylbutane (IUPAC name)
In the IUPAC system of nomenclature, a name is made up of three parts: prefixes, main, and suffixes; The prefixes indicate the substituents of the molecule; the suffix indicates the functional group of the molecule; and the main part the number of carbons it has.
Alkanes can be named following seven steps:
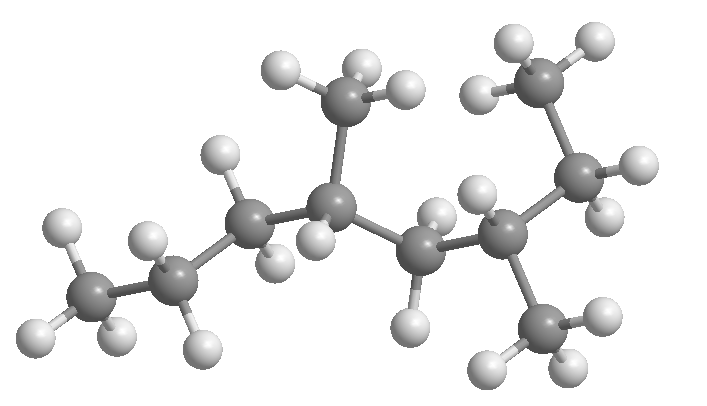
Rule 1.- Determine the number of carbons of the longest chain, called the main chain of the alkane. Observe in the figures that it is not always the horizontal chain.
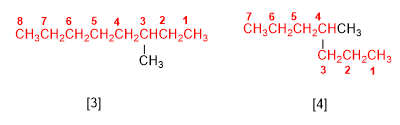
[3] 3-methyloctane
[4] 4-methylheptane
- Details
- Germán Fernández
- ALKANE THEORY
- Hits: 60516
Rule 1. The name of a bicyclic compound is built with the word Bicyclo, followed by a bracket indicating the number of carbons in each of the three chains that start from the bridgehead carbons. The name ends in that of the alkane with the same number of carbons.

[1] Bicyclo[2.2.1]heptane
[2] Bicyclo[3.2.1]octane
[xxx] The bridgehead carbons of the bicyclic compound are drawn with a blue dot. The number of carbons in each of these chains (numbered in red) is indicated within the bracket, without counting the bridgehead carbons. Note that the numbers are ordered from highest to lowest and are separated by periods (not commas).
The name of the bicyclic compound ends in that of the alkane with the same number of carbons.
- Details
- Germán Fernández
- ALKANE THEORY
- Hits: 193560
Methane (CH 4 ), ethane (C 2 H 6 ) and propane (C 3 H 8 ) are alkanes with only one possible structure. However, there are two alkanes with the formula C 4 H 10 ; butane and 2-methylpropane. These alkanes with the same formula but different structures are called isomers.

[1] Butane
[2] 2-Methylpropane
n-Butane and isobutane have the same formula but differ in the way their atoms are joined -they are structural isomers-. Their different structure makes them have different properties, thus, they differ by about 20ºC in their melting point and about 10ºC in their boiling point.
- Details
- Germán Fernández
- ALKANE THEORY
- Hits: 272354
Alkanes are sp3 hybridized compounds on all carbons. The four substituents that start from each carbon are arranged towards the vertices of a tetrahedron.
Bond distances and angles are shown on the following models. 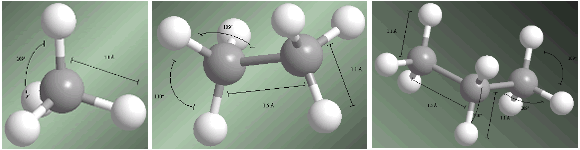
- Details
- Germán Fernández
- ALKANE THEORY
- Hits: 142011
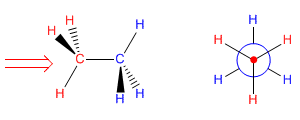 The Newman projection is obtained by looking at the molecule along the CC axis. The front carbon is represented by a point, from which the three bonds that join it to the substituents start. The carbon behind is represented by a circle, and the bonds leaving this carbon are drawn from this circle.
The Newman projection is obtained by looking at the molecule along the CC axis. The front carbon is represented by a point, from which the three bonds that join it to the substituents start. The carbon behind is represented by a circle, and the bonds leaving this carbon are drawn from this circle.
- Details
- Germán Fernández
- ALKANE THEORY
- Hits: 114887
 Single bonds between atoms have cylindrical symmetry and allow rotation of the groups of atoms attached to them. The different spatial arrangements that the atoms adopt as a result of rotation around the bond are called conformations. A particular conformation of many possible ones is called a conformer.
Single bonds between atoms have cylindrical symmetry and allow rotation of the groups of atoms attached to them. The different spatial arrangements that the atoms adopt as a result of rotation around the bond are called conformations. A particular conformation of many possible ones is called a conformer.
- Details
- Germán Fernández
- ALKANE THEORY
- Hits: 111261
Ethane is the simplest alkane that can exist in different conformations. Of the infinite conformations that can be obtained by turning the carbon-carbon bond, the alternated and eclipsed conformations stand out for their importance.
- Details
- Germán Fernández
- ALKANE THEORY
- Hits: 85735
Let's consider the C2-C3 bond of butane. The 60º turns around this link will generate the possible conformations of butane. There are three conformations of special importance called; butane syn , butane anti and butane gauche that we represent in the following models.