 Acetals can be used, due to their stability, as carbonyl protecting groups. The acetal is an ether, very stable in basic media, although it breaks in the presence of acidic media. In many synthesis processes, the carbonyl group is incompatible with the reagent used. In these cases it must be protected to prevent it from reacting. The instability of the acetal in an acidic medium can be used to deprotect the carbonyl.
Acetals can be used, due to their stability, as carbonyl protecting groups. The acetal is an ether, very stable in basic media, although it breaks in the presence of acidic media. In many synthesis processes, the carbonyl group is incompatible with the reagent used. In these cases it must be protected to prevent it from reacting. The instability of the acetal in an acidic medium can be used to deprotect the carbonyl.
Let's see some examples:
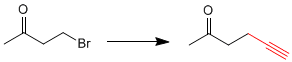
This transformation requires a substitution, using sodium acetylide as the nucleophile. The nucleophile can also attack the carbonyl group, to avoid it we are going to protect it. 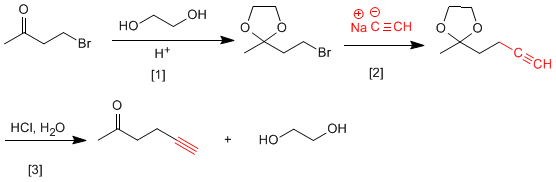
[1] Ketone protection.
[2] Attack of the acetylide on the bromine carbon.
[3] Carbonyl deprotection
Let's look at a second example: 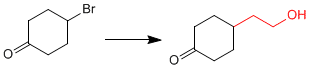
It is necessary to protect the ketone before forming the organometallic to avoid dimerization of the compound. 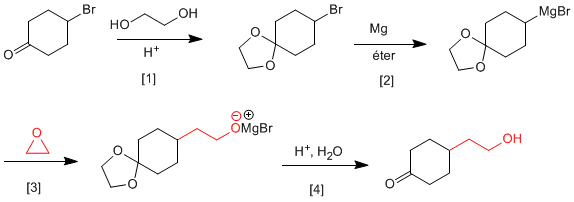
[1] Ketone protection.
[2] Magnesian formation.
[3] Opening of oxacyclopropane.
[4] Deprotection and protonation of the alkoxide.