THEORY OF ALDEHYDES AND KETONES
- Details
- Germán Fernández
- THEORY OF ALDEHYDES AND KETONES
- Hits: 209570
Aldehydes are named by replacing the -e ending of the corresponding alkane with -al . It is not necessary to specify the position of the aldehyde group, since it occupies the end of the chain (locant 1).
When the string contains two aldehyde functions, the suffix -dial is used.
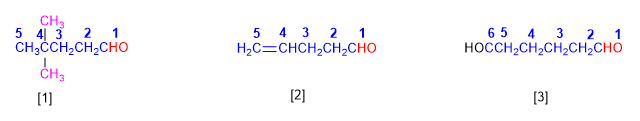
[1] 4,4-Dimethylpentanal
[2] Pent-4-enal
[3] Hexanodial
- Details
- Germán Fernández
- THEORY OF ALDEHYDES AND KETONES
- Hits: 139178
Aldehydes and ketones can be prepared by oxidation of alcohols, ozonolysis of alkenes, hydration of alkynes, and Friedel-Crafts acylation as major methods.
a) Ozonolysis of alkenes: Alkenes break down with ozone to form aldehydes and/or ketones. If the alkene has vinyl hydrogens it gives aldehydes. If it has two carbon chains it forms ketones. 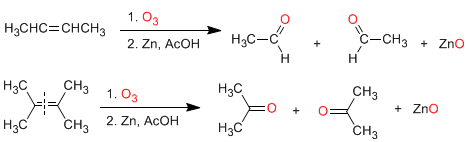
- Details
- Germán Fernández
- THEORY OF ALDEHYDES AND KETONES
- Hits: 68059
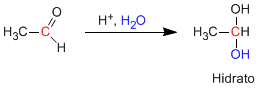
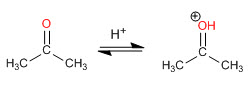
Aldehydes and ketones react in an aqueous acid medium to form hydrates. The mechanism consists of three stages. The first and fastest is the protonation of carbonyl oxygen. This protonation produces an increase in polarity on the carbon and favors the attack of the nucleophile. In the second stage, the water attacks the carbonyl carbon, it is the slow stage of the mechanism. In the third stage, oxygen deprotonation occurs, forming the final hydrate.
- Details
- Germán Fernández
- THEORY OF ALDEHYDES AND KETONES
- Hits: 88053
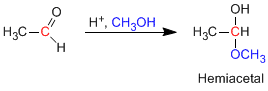
Hemiacetals are formed by reacting one equivalent of alcohol with the carbonyl group of an aldehyde or ketone. This reaction is acid catalyzed and is equivalent to the formation of hydrates.
- Details
- Germán Fernández
- THEORY OF ALDEHYDES AND KETONES
- Hits: 83779
Aldehydes and ketones react with alcohols under conditions of acid catalysis, forming hemiacetals in a first stage, which later evolve by reaction with a second equivalent of alcohol to acetals. 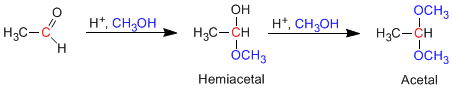
- Details
- Germán Fernández
- THEORY OF ALDEHYDES AND KETONES
- Hits: 59379
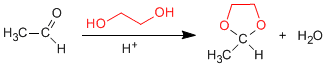
1,2- and 1,3-diols react with aldehydes and ketones to form cyclic acetals. The equilibria are shifted towards the final product by removing the water formed by azeotroping with benzene or toluene.
- Details
- Germán Fernández
- THEORY OF ALDEHYDES AND KETONES
- Hits: 50671
 Acetals can be used, due to their stability, as carbonyl protecting groups. The acetal is an ether, very stable in basic media, although it breaks in the presence of acidic media. In many synthesis processes, the carbonyl group is incompatible with the reagent used. In these cases it must be protected to prevent it from reacting. The instability of the acetal in an acidic medium can be used to deprotect the carbonyl.
Acetals can be used, due to their stability, as carbonyl protecting groups. The acetal is an ether, very stable in basic media, although it breaks in the presence of acidic media. In many synthesis processes, the carbonyl group is incompatible with the reagent used. In these cases it must be protected to prevent it from reacting. The instability of the acetal in an acidic medium can be used to deprotect the carbonyl.
Let's see some examples:
- Details
- Germán Fernández
- THEORY OF ALDEHYDES AND KETONES
- Hits: 78535
The reaction of aldehydes or ketones with primary amines generates imines . The reaction is favored in a slightly acid medium (pH=4.5). 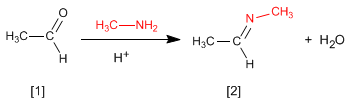
- Details
- Germán Fernández
- THEORY OF ALDEHYDES AND KETONES
- Hits: 58414
oximes are obtained by reaction of aldehydes or ketones and hydroxylamine in a weakly acid medium. The mechanism is analogous to that of imine formation. 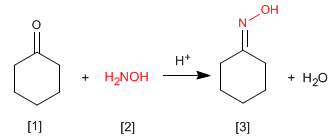
- Details
- Germán Fernández
- THEORY OF ALDEHYDES AND KETONES
- Hits: 53946
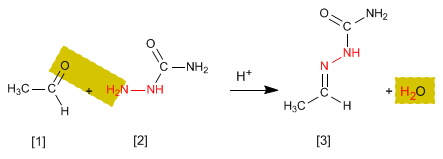
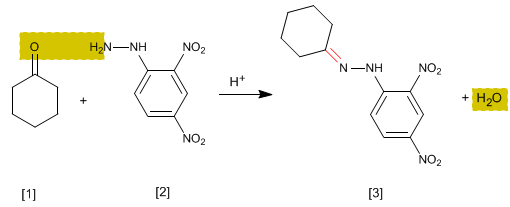
The hydrazones [3] are obtained by reaction of aldehydes or ketones [1] with hydrazine [2]. As in the case of imines and oximes, it requires pH=4. 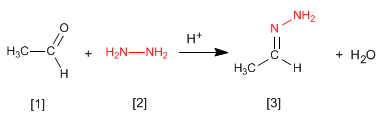
- Details
- Germán Fernández
- THEORY OF ALDEHYDES AND KETONES
- Hits: 30187
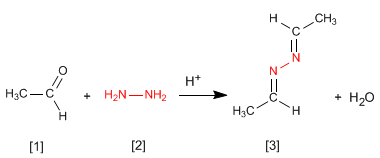
- Details
- Germán Fernández
- THEORY OF ALDEHYDES AND KETONES
- Hits: 34383
- Details
- Germán Fernández
- THEORY OF ALDEHYDES AND KETONES
- Hits: 85631
- Details
- Germán Fernández
- THEORY OF ALDEHYDES AND KETONES
- Hits: 34508
Enamine synthesis
As we saw in previous sections, the condensation of primary amines with aldehydes and ketones generates imines. In this section we will study the condensation of carbonyls with secondary amines that give enamines.
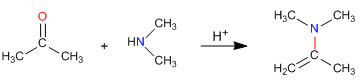
Enamine formation mechanism
After the initial attack of the secondary amine on the carbonyl, water is removed, forming the double bond between the carbonyl carbon and the alpha of the starting carbonyl.
Step 1. Protonation of the carbonyl
Step 2. Nucleophilic attack of the secondary amine
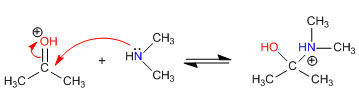
Stage 3. Acid-base balance
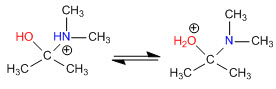
Stage 4. Loss of water
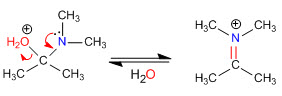
Stage 5. Elimination
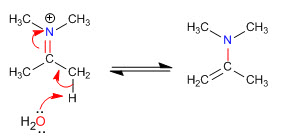
Steric hindrances make the least substituted enamines the most stable
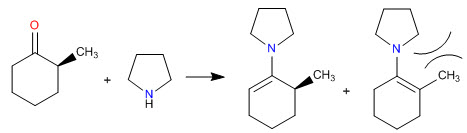
- Details
- Germán Fernández
- THEORY OF ALDEHYDES AND KETONES
- Hits: 56515
Cyanohydrins are formed by reaction of aldehydes or ketones with hydrocyanic acid and are compounds that contain a cynane and a hydroxy group on the same carbon. 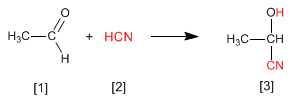
- Details
- Germán Fernández
- THEORY OF ALDEHYDES AND KETONES
- Hits: 63892
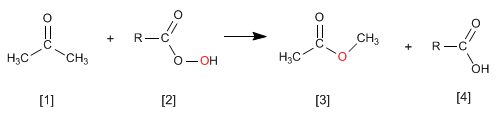
The Wittig reaction employs phosphorus ylides [2] to transform aldehydes and ketones[1] in alkenes [3]. Triphenylphosphine oxide [4] is obtained as a by-product . 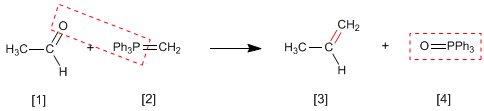
- Details
- Germán Fernández
- THEORY OF ALDEHYDES AND KETONES
- Hits: 57044
The ketone reaction with peracids produces esters . The oxygen of the peracid is inserted between the carbonyl carbon and the alpha carbon of the ketone. This reaction was described by Adolf von Baeyer and Victor Villiger in 1899.